- TEL:
- +81-3-5462-4831
- FAX:
- +81-3-5462-4835
※9:00-17:40 Mon.-Fri. (JST)


Kurniawan DA, Leo S, Inamatsu M, Funaoka S, Aihara T, Mizuno A, Inoue R, Sakura T, Arakawa H, Kato Y, Matsugi T, Esashika K, Shiraki
N, Kume S, Shinha K, Kimura H, Nishikawa M, Sakai Y. PNAS Nexus 3(2): 070, 2024.
https://doi.org/10.1093/pnasnexus/pgae070
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by/4.0/)
In recent years, microphysiological systems (MPS) have attracted attention as an alternative to animal experimentation in drug discovery. Since the year 2022, Sumitomo Bakelite Co., Ltd. has participated in the AMED project for the development of basic technology towards the industrialization of regenerative medicine and gene therapy. We have developed a stirrer pump integrated multi-organ MPS device, an open access cell culture platform which was invented by Professor Hiroshi Kimura of Micro/Nano Technology Center at Tokai University, and have started its test marketing under the brand name of BioStellar™ Plate. Here, we would like to introduce a paper we co-authored with the University of Tokyo using this MPS device.
Intestine and liver are interconnected through enterohepatic circulation, which plays an important role in the metabolism of oral drugs. Coculture of hepatocytes and intestinal cells has shown to increase hepatic drug metabolism, yet its crosstalk mechanism is still unclear. In this study, primary human hepatocytes (PXB cells) from chimeric mouse and human iPS cell-derived small intestinal cells were used to elucidate such crosstalk in this MPS device.
|
As shown in the figure on the right, a stirrer pump (kinetic micro-pump) placed in the microchannel to connecting two compartments provides medium perfusion to the system. PXB cells were seeded in the left compartment and a culture insert containing small intestinal cells was placed in the right to start coculture. The rotation rate was set to adjust the perfusion rate to 35 μL/min. Coculture was performed for 3 days without medium change. The oxygen permeable membrane (PMP), which has low drug adsorption, was used as base for the MPS device to provide increased oxygen supply. ResultsPerfusion and direct oxygenation increased albumin secretion and multiple CYP activities in PXB cells. Barrier integrity of small intestinal cells was also maintained in the coculture system. RNA sequencing results showed upregulated expression of genes related to the arachidonic acid metabolic process in PXB cells cocultured with oxygen supply. Arachidonic acid-treated PXB cells showed increased CPY activity in similar manner as coculture. it is speculated that the release of bile acids from PXB-cells acted as stimuli for iPSc-derived intestine cells to release lipoprotein, which was ultimately taken by PXB-cells and enhanced CYP activity. |
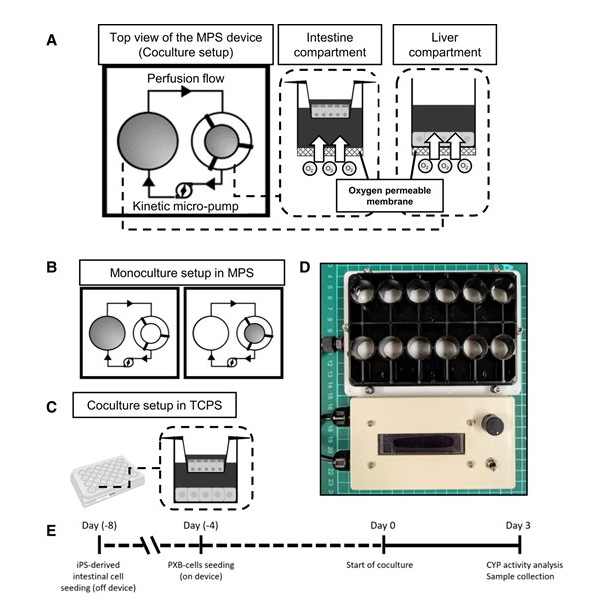
(For details, please refer to the paper) |
|
The modified plate has improved visibility of the fluid path by making it transparent. In addition, the fluid path shape has been redesigned to improve the pumping performance. * The base of this plate has a normal polystyrene bottom and is not equipped with PMP membrane used in the above paper. |

|