July 3, 2024
Tokyo, Japan - July 3, 2024, Sumitomo Bakelite Co., Ltd. (TOKYO: 4203 HQ: Shinagawa-ku, Tokyo, President and Representative Director: Kazuhiko Fujiwara) announces the launch of Antibody Glycan Sample Preparation Kit "Auto-EZGlyco™ mAb-N Kit for SHIMADZU" for Shimadzu's MUP-3100 Automated Sample Preparation Module for Glycan Analysis. Our company aims to develop laboratory automation systems combined with liquid chromatography (LC) and liquid chromatograph-mass spectrometry (LC-MS) in research, development and quality control at pharmaceutical companies and contract development & manufacturing organizations (CDMOs), thereby contributing to reduction of the working time improving the efficiency of glycan analysis of antibody drugs.
Current Status and Issues in Glycan Analysis of Antibody Drugs
Antibody drugs are proteins produced by cell culture using genetic modification technology, and research and development has been actively conducted in recent years. Glycans attached to antibodies are known to affect the efficacy and safety of antibody drugs, hence glycan analysis by LC and LC-MS is necessary in R&D and quality control. Especially in the development of biosimilars, glycan is one of the most important items in drug design and comparative analyses with the original drug are widely conducted by biosimilar manufacturers and CDMOs.
Antibodies secreted by cells usually contain large quantity of contaminants such as proteins other than antibodies, peptides, lipids and other substances derived from cell culture media. Conventionally, glycan analysis can be performed through multiple steps: (1) isolation of antibody from the culture media, (2) glycan liberation from the antibody, (3) purification and (4) fluorescent labeling. However, conventionally, these steps (1) - (4) were often performed separately, requiring total time of about two days and complicated manual works. Therefore, there was a need of time reduction and simplifying the whole process.
To solve these issues, our company released the Antibody Glycan Sample Preparation kit EZGlyco® mAb-N kit with 2-AB in 2016. The kit can complete the above steps (1) - (4) in a few hours with a simple operation, and is widely accepted by many pharmaceutical companies in Japan and overseas. However, since the EZGyco® mAb-N kit with 2-AB is a manual operation kit, as the number of samples increases, the burden on the laboratory operator becomes greater and demand for automation has been increasing.
Auto-EZGlyco™ mAb-N Kit for SHIMADZU
|
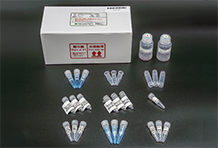
We lauch Antibody Glycan Sample Preparation kit "Auto-EZGlyco® mAb-N Kit for SHIMADZU", a dedicated consumable for MUP-3100, Fully Automated Sample Prepataion Module for Glycan analysis manufactured by Shimadzu Corporation. MUP-3100 is a device that properly and automatically replicates the manual operations of the EZGlyco® mAb-N kit with 2 AB. It automates the sample preparation steps of glycan analysis using LC and LC-MS in R&D and quality control of antibody drugs, helping to reduce not only the burden on laboratory workers but also human-caused procedural errors, poor analysis results, and data variations. |
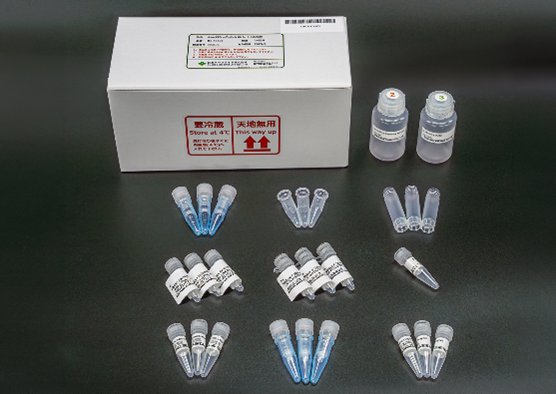
Auto-EZGlyco® mAb-N Kit for SHIMADZU |
Future Business Plan
Our company plan an expansion of sales of glycan analysis products in collaboration with Shimadzu. By combining our glycan preparation technology with Shimadzu's automation equipment and analysis system (LC, LC-MS), we will establish a new solution and aim to expand business in the antibody drug glycan analysis market. We aim to expand sales not only in Japan but also in Asia, where the biosimilar industry and CMOs/CDMOs are growing rapidly.
What are Glycans?
Glycans are one of the important biological biomolecules that constitute living organisms including us humans. Most of proteins are modified with glycans, which control the properties and functions of proteins. In case of antibody drugs, glycans are known to be related with antibody-dependent cellular cytotoxicity (ADCC) and immunogenicity.
Reference: MUP-3100, Fully Automated Sample Prepataion Module for Glycan Analysis

MUP-3100, Fully Automated Sample Preparation Module for Glycan Analysis, manufactured by Shimadzu Corporation |
SHIMADZU CORPORATION |
Related Information
For inquiries
S-BIO Business Division, Sumitomo Bakelite Co., Ltd.
TEL: +81-3-5462-4831